R Bio re-applied for 토토 바카라 this month; approval expected in autumn
Discussion ongoing on rejection by Central Pharmaceutical Affairs Council
Supplementary 토토 바카라 confirms efficacy and statistical/clinical significance.
R Bio, a subsidiary of Nature Cell, has resubmitted its application for product approval for the stem cell therapy '토토 바카라' earlier this month. This follows their initial application to the Ministry of Food and Drug Safety (MFDS) in 2021, marking a three-year journey since the first submission. Notably, it has been a year since the rejection of the application in April 2023.
The rejection of the 토토 바카라 application in 2023 sent ripples through the pharmaceutical bioindustry. The reason cited for rejection was the lack of "clinical significance" despite statistical significance, raising questions within the industry about the standards for approval. Revisiting the events surrounding this rejection is crucial to understanding the dynamics at play.
With the re-application now underway, a critical examination of the new data presented by R Bio is imperative. This fresh submission holds the potential to shed light on the prospects of 토토 바카라 based on the updated information provided.
Reason for Rejection of 토토 바카라’s Approval
토토 바카라, designed as a treatment for knee osteoarthritis, aims to alleviate knee pain and improve mobility. To establish its efficacy, R Bio employed the 'WOMAC' and 'VAS' indices during the Phase 3 clinical trial. These indices measured joint function and pain levels before and after the administration of 토토 바카라 injections.
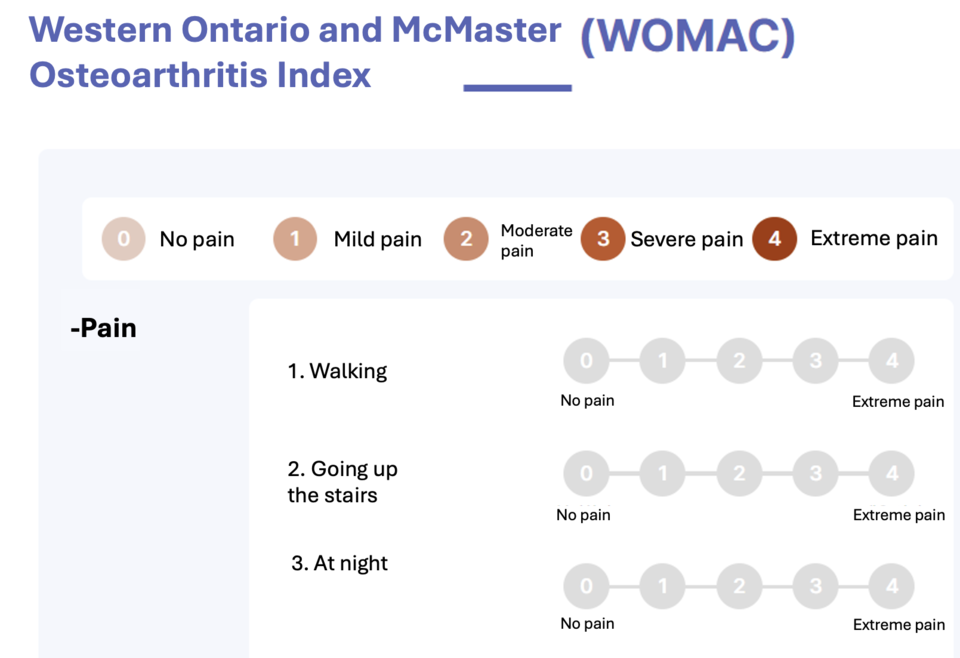
Upon analysis, significant improvements in scores post-injection were statistically evident. This formed the basis of R Bio's application for MFDS approval in 2021. Initial review by the Central Pharmaceutical Affairs Council (CPAC) deemed 토토 바카라 statistically and clinically significant. However, a subsequent meeting reversed this opinion, emphasizing statistical significance but questioning its clinical relevance, ultimately leading to rejection.
The notion of "clinical insignificance" highlights a disparity between statistical effects and meaningful clinical outcomes. This disparity was underscored during the CPAC's second meeting, where the discussion veered away from predefined criteria, focusing instead on predetermined thresholds set by R Bio. Such deviations from objective evaluation standards fueled widespread criticism of the rejection decision.
It's akin to expecting a perfect score on a test, only to achieve a 90. Instead of assessing against established criteria, the teacher opts to fail you for falling short of your own 100-point expectation. This scenario appears unjust and arbitrary, as the evaluation hinges not on objective standards but on personal expectations.

Additionally, speculative discussions during the meeting regarding 토토 바카라's mechanism of action and its comparison to alternative therapies further fueled controversy. Criticisms arose over the lack of concrete evidence supporting these assertions.
Despite these setbacks, R Bio has embarked on a renewed effort to secure approval for 토토 바카라. The analysis of their supplementary data submission offers insights into the ongoing quest for regulatory acceptance.
Key Supplementary Data Supporting 토토 바카라’s Re-Approval
In its re-application for 토토 바카라 approval, R Bio presents crucial supplementary data gathered over the past three years. These long-term follow-up results from Phase 3 clinical trials bolster the case for re-approval, showcasing the sustained efficacy of the treatment.
To grasp the significance of this three-year data, it's essential to understand the current landscape of knee osteoarthritis treatment. Existing methods, such as NSAIDs, steroid injections, hyaluronic acid injections, and joint replacement surgery, have notable limitations. 토토 바카라 aims to surpass these limitations by offering sustained pain relief for more than six months with a single administration, accompanied by fewer side effects and clinically significant results.
The analysis of WOMAC and VAS scores over the three-year period reveals sustained improvements in 토토 바카라 function and pain reduction post-administration, demonstrating statistical significance.
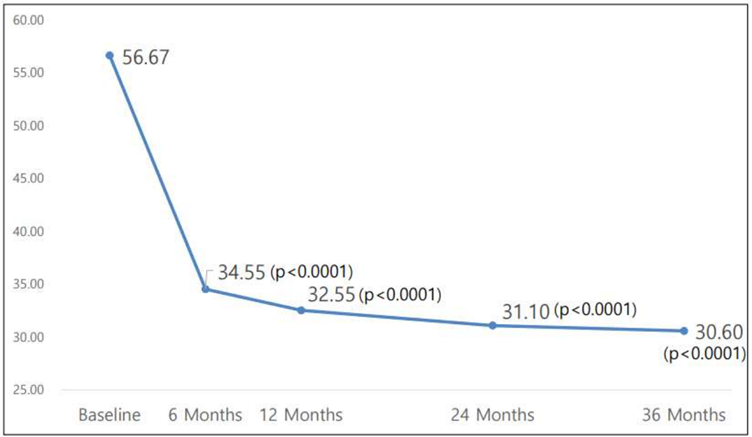
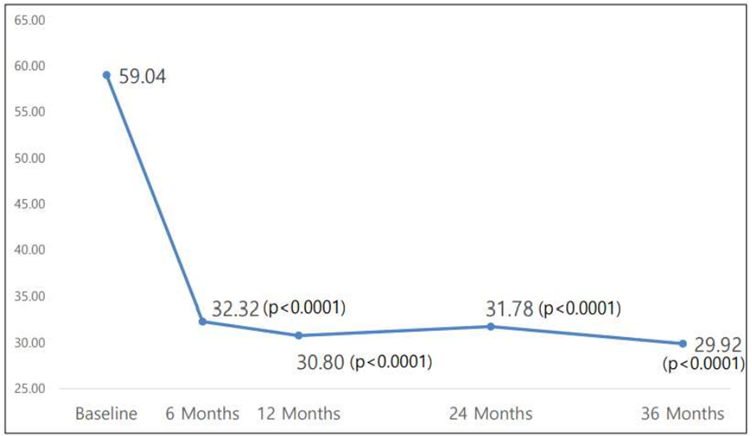
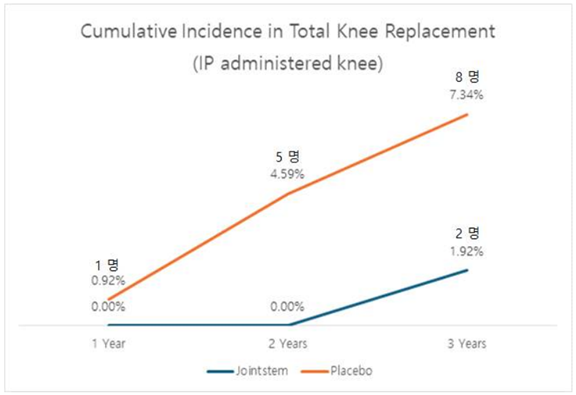
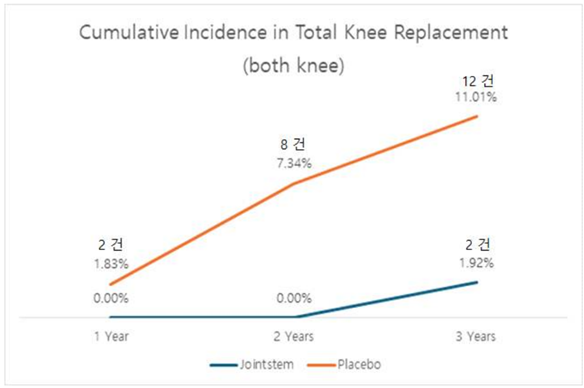
Additionally, concerns raised during previous CPAC meetings regarding 토토 바카라's ability to reduce the need for joint replacement surgery are addressed. Data analysis indicates a significant reduction in the number of patients requiring joint replacement surgery after receiving 토토 바카라 compared to those receiving the control drug.
JoinStem: Prospects for 토토 바카라
R Bio's presentation of three-year follow-up data underscores the efficacy and safety of 토토 바카라, potentially paving the way for its approval. The treatment's ability to provide long-term pain relief without significant side effects, coupled with a reduction in the need for joint replacement surgeries, addresses previous concerns raised during the rejection.
However, uncertainties linger regarding CPAC's response this time around. Clear quantitative criteria and the involvement of knee osteoarthritis specialists in CPAC meetings would be pivotal in ensuring a fair evaluation.
As the decision on 토토 바카라's approval approaches in approximately six months, further discussions will ensue in autumn, revisiting pertinent issues at that time.
